四、第二代TKI的选择
目前国内可供选择的第二代TKI为尼洛替尼以及达沙替尼,二者对不同分期CML患者治疗效果相似,但二者具有显著不同的药代动力学、药物相互作用以及不良反应,二者的选择可参照如下原则[9,13,14,15,24,30,50,51,52]:
1.应综合考虑患者病史、合并症、合并用药、药物不良反应以及药物说明书并结合BCR-ABL激酶区突变类型选择。
2.参照BCR-ABL激酶区突变类型:目前以下7种类型突变对于达沙替尼或尼洛替尼选择具有较为明确的指导意义。
①T315I:二者均耐药,有条件者可进入临床试验,或选择恰当的治疗方案;
②F317L/V/I/C、V299L、T315A:采用尼洛替尼治疗更易获得临床疗效;
③Y253H、E255K/V、F359C/V/I:采用达沙替尼治疗更易获得临床疗效。
五、allo-HSCT在CML中应用
allo-HSCT依然是CML治疗的重要手段,尤其是TKI耐药以及进展期患者。在TKI治疗时代移植不再是CML慢性期患者的一线治疗选择,原则上对至少1种第二代TKI不耐受或耐药的患者考虑allo-HSCT。目标人群包括:①对于标准的伊马替尼治疗失败的慢性期患者,可根据患者的年龄和意愿考虑行HSCT。②治疗任何时候出现ABL基因T315I突变的患者,首选HSCT。③对第二代TKI治疗反应欠佳、失败或不耐受的所有患者。④更换第二代TKI 6个月后仍未获得主要细胞遗传学反应者,其12个月获得次要细胞遗传学反应以及长生存的可能性明显降低,应尽早考虑HSCT。⑤加速期或急变期患者。
移植供者的选择、预处理方案以及移植后监测参考《中国慢性髓性白血病诊断与治疗指南(2013年版)》[53]。
六、TKI治疗期间的妊娠管理
参考《中国慢性髓性白血病诊断与治疗指南(2013年版)》[53]。
(执笔:刘兵城)
参与指南讨论的专家:中国医学科学院血液学研究所血液病医院(王建祥、韩明哲、刘兵城);上海交通大学医学院附属瑞金医院(沈志祥、李军民);北京大学人民医院血液病研究所(黄晓军、江倩);四川大学华西医院(刘霆);第二军医大学附属长海医院(王健民);福建医科大学附属协和医院(胡建达);中国医学科学院北京协和医院(赵永强);哈尔滨血液病肿瘤研究所(马军);贵州医学院附属医院(王季石);山东省立医院(王欣);上海交通大学医学院附属第一人民医院(王椿);安徽省立医院(孙自敏);江苏省人民医院(李建勇);中山大学附属第一医院(李娟);南方医科大学南方医院(刘启发);河南省肿瘤医院(宋永平);第四军医大学附属西京医院(陈协群);广东省人民医院(杜欣);南昌大学第一附属医院(陈国安);第三军医大学附属新桥医院(张曦);山西医科大学第二医院(杨林花);华中科技大学同济医学院附属协和医院(邹萍、胡豫);苏州大学附属第一医院(吴德沛);浙江大学医学院附属第一医院(金洁、黄河);山东大学齐鲁医院(侯明);第四军医大学附属唐都医院(梁英民);广西医科大学附属第一医院(赖永榕);中南大学湘雅第二医院(张广森);河北医科大学第二医院(罗建民);中国医科大学附属第一医院(李艳);中国医科大学附属盛京医院(刘卓刚);华中科技大学同济医学院附属同济医院(周剑峰);解放军总医院(于力);深圳市第二人民医院(杜新)
参考文献
[2]DrukerBJ, LeeSJ. Chapter 43: Chronic Leukemias: Section 1: Chronic Myelogenous Leukemia//Cancer Principles and Practice of Oncology. 2007.
[3]全国白血病与再生障碍性贫血流行病学调查协作组.全国白血病发病情况调查[J].中国医学科学院学报, 1992,14(2):12–18.
[4]贺其图,时风桐,袁祖正,等.包头市白血病流行病学调查[J].内蒙古医学杂志, 1993, 13(2):3–5.
[5]张新友,张大龙,孙璇,等.深圳特区白血病与再生障碍性贫血的流行病学调查[J].中华血液学杂志, 2001,22(7):347. .
[6]胡进林,冒镇,董德平,等.海安县15年白血病流行病学调查[J].中国交通医学杂志, 2004, 18(1):114–115..
[7]唐正贤,孙秋云,张金桃,等.上海市金山县11年白血病流行病学调查[J].中华血液学杂志, 1994, 15(8): 430.
[8]KalmantiL, SausseleS, LausekerM, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML- study IV [J]. Leukemia, 2015, 29 (5):1123–1132. .
[9]LarsonRA, HochhausA, HughesTP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up[J]. Leukemia, 2012, 26(10):2197–2203. .
[10]WangJ, ShenZX, SaglioG, et al. Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina [J]. Blood, 2015,125(18):2771–2778. .
[11]CortesJE, SaglioG, KantarjianHM, et al. Final 5- Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment- Naïve Chronic Myeloid Leukemia Patients Trial [J]. J Clin Oncol,2016, 34 (20):2333–2340. .
[12]HughesTP, RossDM. Moving treatment- free remission into mainstream clinical practice in CML[J].Blood, 2016, 128(1): 17–23. .
[14]BaccaraniM, PileriS, SteegmannJL, et al. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [J]. Ann Oncol, 2012, 23Suppl 7:vii72–77. .
[15]BaccaraniM, DeiningerMW, RostiG, et al. European Leukemi-aNet recommendations for the management of chronic myeloid leukemia: 2013[J]. Blood, 2013, 122(6):872–884. .
[16]World Health Organization Classification of Tumors. Pathology and Genetic of Tumors of Haematopoietic and Lymphoid Tissue. 2008.
[17]SokalJE, CoxEB, BaccaraniM, et al. Prognostic discrimination in "good- risk" chronic granulocytic leukemia[J]. Blood, 1984, 63(4):789–799.
[18]HasfordJ, PfirrmannM, HehlmannR, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group [J]. J Natl Cancer Inst, 1998, 90(11):850–858.
[19]HasfordJ, BaccaraniM, HoffmannV, et al. Predicting complete cytogenetic response and subsequent progression- free survival in 2060 patients with CML on imatinib treatment: the EUTOS score [J].Blood, 2011, 118 (3):686–692. .
[20]江倩,陈珊珊,江滨,等.甲磺酸伊马替尼治疗慢性粒细胞白血病慢性期100例追踪观察[J].中华血液学杂志,2006, 27(11): 721–726. .
[21]王国蓉,赵耀中,钱林生,等.伊马替尼治疗95例慢性粒细胞白血病的远期疗效及其影响因素分析[J].中华血液学杂志, 2008, 29(1):18–22. .
[22]周励,王爱华,王黎,等.伊马替尼治疗慢性粒细胞白血病151例临床疗效及安全性观察[J].中华血液学杂志,2008, 29(1): 13–17. .
[23]SchifferCA. BCR- ABL tyrosine kinase inhibitors for chronic myelogenous leukemia [J]. N Engl J Med,2007, 357(3):258–265. .
[24]PavlovskyC, KantarjianH, CortesJE. First- line therapy for chronic myeloid leukemia: Past, present, and future [J]. Am J Hematol, 2009, 84(5):287–293. .
[25]KantarjianHM, CortesJ, La RoséeP, et al. Optimizing therapy for patients with chronic myelogenous leukemia in chronic phase [J]. Cancer, 2010, 116(6):1419–1430. .
[26]ValentP, HadzijusufovicE, SchernthanerGH, et al. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors[J]. Blood, 2015, 125(6):901–906. .
[27]MarinD, IbrahimAR, LucasC, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors[J]. J Clin Oncol, 2012,30(3):232–238. .
[28]HanfsteinB, MüllerMC, HehlmannR, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) [J]. Leukemia,2012, 26 (9):2096–2102. .
[29]GargRJ, KantarjianH, O'BrienS, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow- up [J]. Blood, 2009, 114 (20):4361–4368. .
[30]JabbourE, JonesD, KantarjianHM, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations [J]. Blood,2009,114(10):2037–2043. .
[31]AlvaradoY, KantarjianH, O'BrienS, et al. Significance of suboptimal response to imatinib, as defined by the European LeukemiaNet, in the long- term outcome of patients with early chronic myeloid leukemia in chronic phase[J]. Cancer, 2009,115(16):3709–3718. .
[32]HochhausA, BaccaraniM, DeiningerM, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib[J]. Leukemia, 2008, 22(6):1200–1206. .
[33]GargRJ, KantarjianH, O'BrienS, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow- up [J]. Blood, 2009, 114 (20):4361–4368. .
[34]KantarjianH, GilesF, WunderleL, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome- positive ALL [J]. N Engl J Med, 2006, 354 (24):2542–2551. .
[35]JabbourE, CortesJ, SantosFP, et al. Results of allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia patients who failed tyrosine kinase inhibitors after developing BCR- ABL1 kinase domain mutations [J]. Blood, 2011, 117(13):3641–3647. .
[36]SausseleS, LausekerM, GratwohlA, et al. Allogeneic hemato-poietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV [J]. Blood, 2010, 115(10):1880–1885. .
[37]MarinD, BazeosA, MahonFX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib [J]. J Clin Oncol, 2010, 28 (14):2381–2388. .
[38]O'BrienSG, GuilhotF, LarsonRA, et al. Imatinib compared with interferon and low- dose cytarabine for newly diagnosed chronic- phase chronic myeloid leukemia [J]. N Engl J Med, 2003,348(11):994–1004. .
[39]PreudhommeC, GuilhotJ, NicoliniFE, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia[J]. N Engl J Med, 2010, 363(26):2511–2521. .
[40]OhanianM, KantarjianHM, Quintas-CardamaA, et al. Tyrosine kinase inhibitors as initial therapy for patients with chronic myeloid leukemia in accelerated phase [J]. Clin Lymphoma Myeloma Leuk,2014,14 (2):155–162.e1. .
[41]de LabartheA, RousselotP, Huguet- RigalF, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome- positive acute lymphoblastic leukemia: results of the GRAAPH- 2003 study [J]. Blood, 2007, 109(4):1408–1413. .
[42]CortesJ, KimDW, RaffouxE, et al. Efficacy and safety of dasat-inib in imatinib- resistant or - intolerant patients with chronic myeloid leukemia in blast phase [J]. Leukemia, 2008, 22(12):2176–2183. .
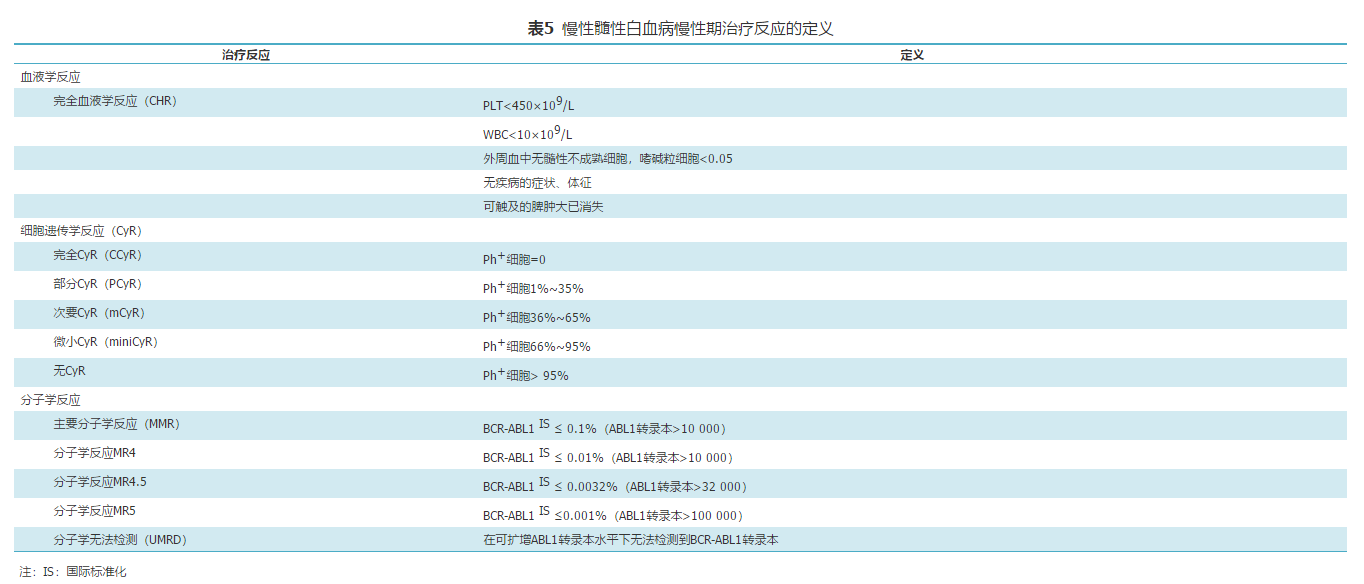
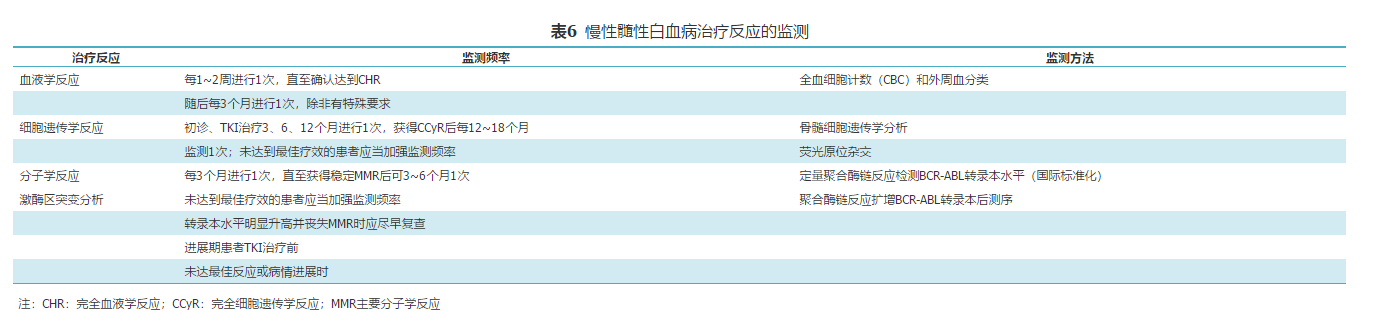
[43]中华医学会血液学分会实验诊断学组,中国慢性髓性白血病联盟专家组.中国慢性髓性白血病诊疗监测规范(2014年版) [J].中华血液学杂志, 2014, 35(8): 781–784. .
[44]KantarjianH, SchifferC, JonesD, et al. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods [J]. Blood, 2008, 111(4):1774–1780. .
[45]LandstromAP, TefferiA. Fluorescent in situ hybridization in the diagnosis, prognosis, and treatment monitoring of chronic myeloid leukemia [J]. Leuk Lymphoma, 2006, 47(3):397–402. .
[46]HughesT, DeiningerM, HochhausA, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR- ABL transcripts and kinase domain mutations and for expressing results [J]. Blood,2006, 108(1):28–37. .
[47]BranfordS, CrossNC, HochhausA, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia [J].Leukemia, 2006, 20 (11):1925–1930. .
[48]BranfordS, RudzkiZ, ParkinsonI, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR- ABL kinase domain mutations [J]. Blood, 2004, 104(9):2926–2932. .
[49]DewaldGW, WyattWA, JuneauAL, et al. Highly sensitive fluorescence in situ hybridization method to detect double BCR/ABL fusion and monitor response to therapy in chronic myeloid leukemia[J].Blood, 1998, 91(9):3357–3365.
[50]DoggrellSA, ChristensenAM. Are there better Bcr- Abl kinase inhibitors for chronic myeloid leukaemia than imatinib? Evaluation of Saglio G, Kim D-W, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362:2251-9, and Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362:2260- 70 [J]. Expert Opin Pharmacother,2011, 12 (1):157–163. .
[51]HaoualaA, WidmerN, DuchosalMA, et al. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib [J]. Blood, 2011, 117(8):e75–87. .
[52]SoveriniS, HochhausA, NicoliniFE, et al. BCR- ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet[J]. Blood, 2011, 118(5):1208–1215. .
[53]中华医学会血液学分会.中国慢性髓性白血病诊断与治疗指南(2013年版)[J].中华血液学杂志, 2013, 34(5):464–470. .